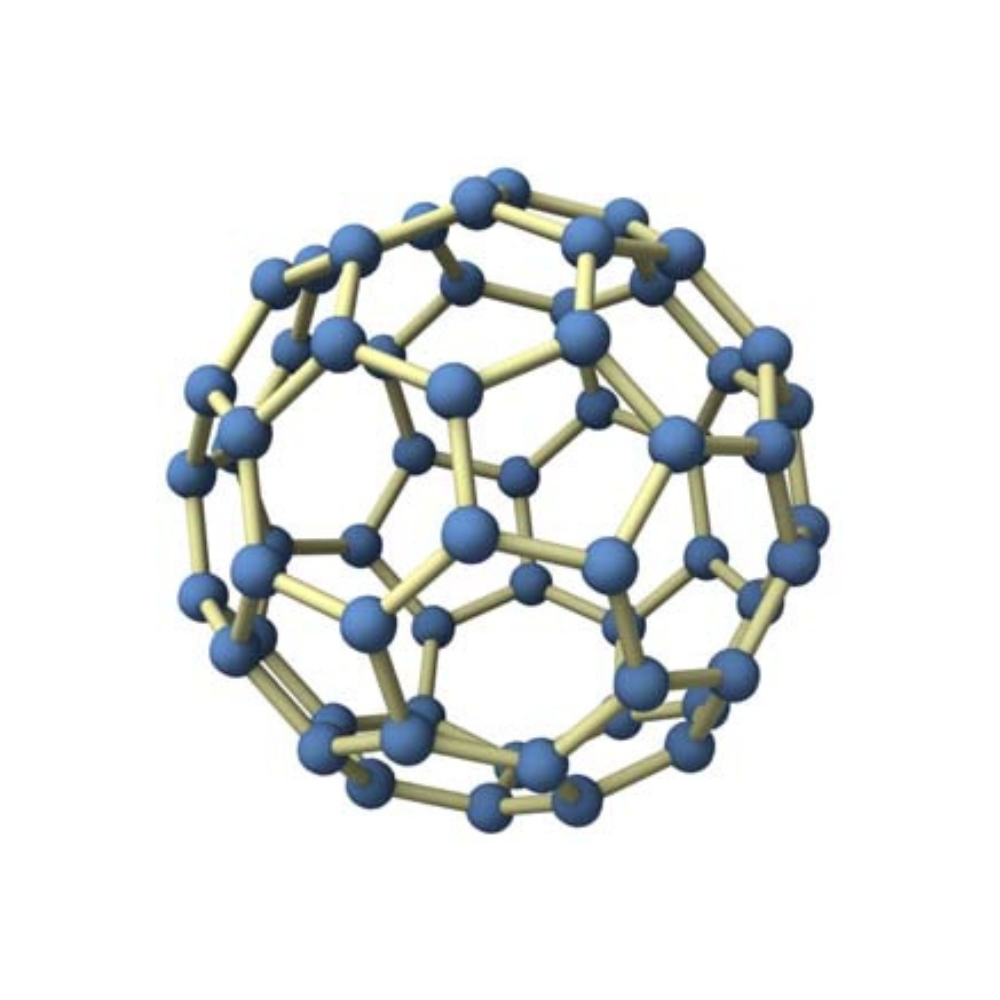
The chemical formula C60 represents a molecule called Buckminsterfullerene or simply Fullerene.
It is a type of carbon molecule with a hollow spherical shape, similar to a soccer ball, consisting of 60 carbon atoms arranged in a pattern of pentagons and hexagons.
Fullerene was discovered in 1985 by three scientists named Harold Kroto, Robert Curl, and Richard Smalley, who were awarded the Nobel Prize in Chemistry in 1996 for their discovery.
What are the properties of Buckminsterfullerene?
Buckminsterfullerene, also known as C60 or fullerene, has some unique and interesting properties. Here are a few of them:
-
Molecular structure: Buckminsterfullerene has a spherical shape, consisting of 60 carbon atoms arranged in a pattern of 12 pentagons and 20 hexagons, similar to a soccer ball.
-
Carbon bonds: The carbon atoms in Buckminsterfullerene are bonded covalently, which means they share electrons to form strong bonds.
-
High stability: Buckminsterfullerene is highly stable due to its spherical shape and strong covalent bonds. It can withstand high temperatures, pressures, and chemical reactions.
-
Conductivity: Buckminsterfullerene has some electrical conductivity due to the delocalization of its electrons. It can conduct both electricity and heat.
-
Photovoltaic properties: Buckminsterfullerene has photovoltaic properties, meaning it can absorb light and convert it into electrical energy.
-
Antioxidant properties: Buckminsterfullerene has been shown to have antioxidant properties, meaning it can neutralize free radicals in the body and protect against oxidative stress.
-
Solubility: Buckminsterfullerene is insoluble in water but can dissolve in some organic solvents.
These unique properties make Buckminsterfullerene an interesting and versatile material with potential applications in various fields, including electronics, medicine, and energy production.
How is Buckminsterfullerene synthesized?
Buckminsterfullerene, or C60, can be synthesized using a process called arc discharge or the Krätschmer-Huffman method.
Here are the steps involved in this process:
Warning: *Do not attempt to replicate this process this is for informational use only*
-
Set up an apparatus: The process begins by setting up an apparatus consisting of two electrodes in a vacuum chamber. One electrode is made of graphite, and the other is made of metal.
-
Create an arc: A high voltage is applied between the two electrodes, creating an electric arc that vaporizes the graphite electrode.
-
Collect the vaporized carbon: The vaporized carbon condenses on a cooler surface at the other end of the chamber, forming a sooty residue.
-
Extract Buckminsterfullerene: The sooty residue is then extracted using solvents and separated into different fractions based on their molecular weight. The Buckminsterfullerene molecules are separated using a process called chromatography.
-
Purify Buckminsterfullerene: The purified Buckminsterfullerene can then be further processed using various methods to remove impurities and improve its quality.
There are also other methods of synthesizing Buckminsterfullerene, including laser vaporization and chemical vapor deposition, but the arc discharge method is the most commonly used.