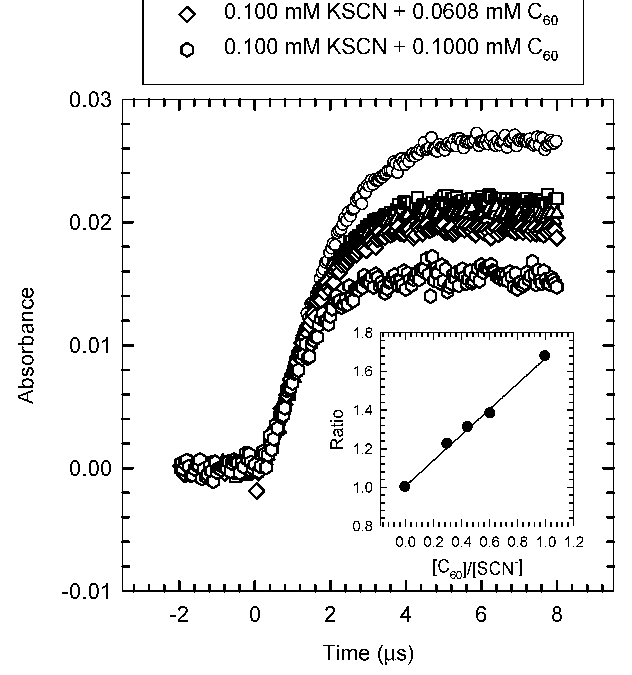
Quantum mechanical calculations of binding energy of the C60−OH adduct as a function of C60 clustering degree indicate, despite an initial fast reaction, a slower overall conversion due to thermodynamic instability of C60−OH intermediates. The results imply that ordered clustering of C60 in the aqueous phase significantly hinders C60’s fundamental reactivity with radical species. Download the PDF
Antioxidants support your immune system and fight disease including viruses like the flu.
Enjoy one of the world’s most effective antioxidants for $1/day.
Science Blog
- Allergic Response (1)
- Alzheimer's (1)
- Anti-inflammatory (5)
- Antioxidant (15)
- C60 Top Research (14)
- Carbon C60 (122)
- Cardiovascular Disease (2)
- Energy (1)
- Free Radicals (3)
- Immune system (3)
- Longevity (4)
- Mental Focus (2)
- News (6)
- Resources (2)
- Reviews & Testimonials (10)
- Sleep (2)
- Sun Burn (1)
- Toxicity (7)
- Uncategorized (9)